BBMRI-ERIC faces the challenge that human biological samples are finite resources and providing access is subject to a series of ethical and legal restrictions, thus requiring innovative solutions for efficient utilisation. Therefore, BBMRI-ERIC developed the concept of Expert Centres as public-private partnerships in the precompetitive, non-profit field to provide a new structure to perform research projects under a novel model of academic-industry collaboration.
BBMRI-ERIC Expert Centres function as a common entity of the public and the private sectors (Fig. 2).
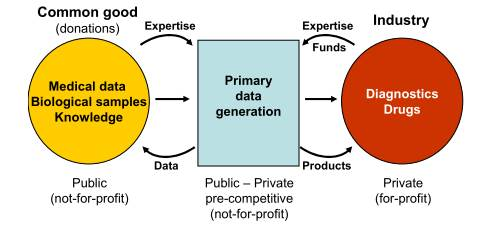
The private industry sector needs access to human biospecimen and medical data to develop innovative products, such as biomarkers and drugs to keep or gain market leadership. Human biological samples and medical data are provided as donations and are considered as common goods, and commercialization of human bodily materials is forbidden according to the European Oviedo Convention (ETS 164) and by national legislation in most Member States. In addition, providing access to these resources on a cost recovery basis is difficult because of the complex stakeholder situation (e.g. patients, medical doctors, hospitals, universities, funders). Therefore engaging academia and industry in research collaboration, for which Expert Centres provide a proper framework, is a solution to overcome the hurdles.
The concept of the Expert Centres associated with BBMRI-ERIC foresees the primary analysis of quality defined human biological samples using the latest analytical technology and standardized procedures. This should result in best possible usage of finite resources by the transformation of biological samples into data and knowledge that can be shared and jointly used by academia and industry. Such a quality-defined knowledge base will enhance academic research and lay the foundation for new product development by industry.
BBMRI-ERIC associated Expert Centres are characterised by:
- Access to quality-defined human biological samples and medical data,
- State-of-the-art banking and analytical technologies,
- High level of standardization,
- Professional quality management,
- International interoperability,
- Cost efficiency,
- Confidentiality,
- Flexible solutions for generation of intellectual property,
- Ethical and legal compliance.
BBMRI-ERIC associated Expert Centres can be established in different countries. Since Expert Centres have to implement common quality standards and participate in common proficiency testing, data generated in different Expert Centres should be well suited for integrated data analysis, which would also allow the analysis of samples in the country of origin, avoiding the need for transnational sample shipment in international collaboration. This is of particular relevance for global research collaboration since many countries have posed severe restrictions on sample shipment.
Reference:
van Ommen GJ, Törnwall O, Bréchot C, Dagher G, Galli J, Hveem K, Landegren U, Luchinat C, Metspalu A, Nilsson C, Solesvik OV, Perola M, Litton JE, Zatloukal K. BBMRI-ERIC as a resource for pharmaceutical and life science industries: the development of biobank-based Expert Centres. Eur J Hum Genet. 2015 Jul;23(7):893-90